Abstract
Background: Autologous hematopoietic stem cell transplantation (autoHSCT) following response to salvage chemotherapy is the standard of care for patients with relapsed/refractory (r/r) aggressive B-cell lymphoma after first-line chemotherapy. Patients without complete metabolic response (CMR) to first salvage (S1) therapy have limited options and poor outcomes based on historical data. Blinatumomab, a bispecific T-cell engager (BiTE®) antibody construct that directs cytotoxic T cells to lyse B cells expressing CD19, has demonstrated a survival benefit in B-cell acute lymphoblastic leukemia and has antitumor activity in patients with r/r aggressive B-cell non-Hodgkin lymphoma (B-NHL), including patients previously treated with autoHSCT. This open-label, multicenter, phase 2 portion of an adaptive phase 2/3 study (ClinicalTrials.gov, NCT02910063) assessed the efficacy and safety of blinatumomab as a second salvage (S2) therapy for patients with aggressive r/r NHL who have not achieved CMR following platinum-based S1 chemotherapy.
Methods: Patients ≥18 years had biopsy-confirmed r/r aggressive B-NHL without a prior complete remission or CMR after first-line treatment with an anthracycline and anti-CD20 agent, and had either progressive metabolic disease (PMD), no metabolic response (NMR), or partial metabolic response (PMR; Lugano Classification) after ≥2 cycles of platinum-based S1 therapy. Patients with prior radiotherapy were PET+ ≥6 weeks after the last dose. Blinatumomab was given by continuous intravenous infusion for a single 70-day cycle 1 (9 μg/day for 7 days, 28 μg/day for 7 days, and 112 μg/day for 42 days, followed by a 14-day treatment-free interval) and an optional 28-day cycle 2 (9 μg/day for 7 days, 28 μg/day for 7 days, and 112 μg/day for 14 days). The primary endpoint was CMR by central PET. Additional endpoints were objective response rate (ORR [CMR + PMR]), post-response HSCT realization rates, and the incidence/severity of adverse events (AEs).
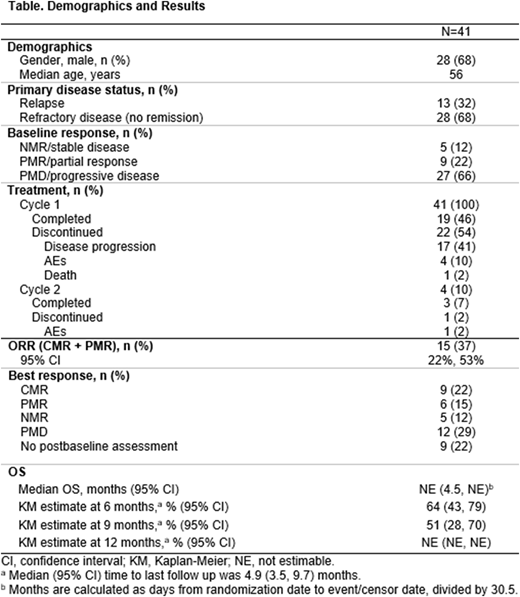
Results: Of the 41 patients enrolled, most had PMD/progressive disease (66%) and had refractory (68%) or relapsed (32%) disease; 5 (12%) had NMR, and 9 (22%) had PMR (Table). All 41 patients received blinatumomab; 19 (46%) completed cycle 1 (Table). Twenty-two patients discontinued cycle 1 (disease progression, n=17; AE n=4; death, n=1). Among the 17 patients who discontinued cycle 1 due to disease progression, 8 (47%) completed at least 90% of planned treatment duration. Four patients started cycle 2; 3 (7%) completed cycle 2. One patient discontinued cycle 2 due to AEs. The ORR (within 12 weeks of starting blinatumomab) was 37% (15/41 patients; 95% CI, 22%, 53%); 9 (22%) patients achieved CMR (Table). Eight (20%) patients had HSCT in remission, 7 (17%) with autoHSCT (CMR, n=6; PMR, n=1), and 1 with allogeneic HSCT in PMR. Thirty-five patients did not have HSCT (n=32) or had delayed HSCT (n=3) due to PMD (n=17), lack of CMR (n=4), AE (n=4), patient preference (n=1), NMR or unknown (n=1), and other (n=8); 1 patient had missing information. Eight of 9 CMR patients (89%) were alive without relapse, with a median follow up time of 8.8 months. The Kaplan-Meier estimate at 9 months was 51%; median overall survival (OS) was not reached (Table).
In total, 24 (59%) patients had grade ≥3 treatment-emergent AEs, and 10 (24%) had grade ≥4 treatment-emergent AEs. Seven (17%) patients discontinued treatment due to AEs. Consistent with previous blinatumomab reports, neurologic events (NEs) were reported in 23 (56%) patients, including 10 (24%) with grade 3 NEs and 3 (7%) with NEs leading to treatment discontinuation. Grade 3 cytokine release syndrome was reported in only 1 patient. Other grade ≥3 AEs included infections (n=8; 20%), bone marrow toxicity (n=7; 17%), thromboembolic events (n=3; 7%), hepatic disorders (n=2; 5%), and acute pancreatitis (n=1; 2%).
Conclusions: In patients with r/r aggressive B-NHL and predominantly progressive disease following ≥2 cycles of platinum-based S1 chemotherapy, blinatumomab monotherapy as S2 therapy induced CMR/PMR in 37% of patients and led to HSCT in 20%. When considering that 66% of the patients enrolled had progressive disease and that 47% received the therapeutic dose, blinatumomab showed promising efficacy consistent with the efficacy and safety demonstrated in earlier blinatumomab B-NHL trials and potentially offers a treatment option for patients unresponsive to standard salvage regimens.
Coyle:Amgen Inc.: Other: non-financial relationship. Morley:Amgen Inc.: Honoraria, Other: non-financial; Roche: Honoraria, Other: non-financial, Research Funding. Rambaldi:Celgene: Consultancy; Roche: Consultancy; Omeros: Consultancy; Italfarmaco: Consultancy; Novartis: Consultancy; Amgen Inc.: Consultancy; Pfizer: Consultancy. Zhang:Amgen Inc.: Employment, Equity Ownership. Jung:Amgen Inc.: Employment, Equity Ownership. Franklin:Amgen Inc.: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.